For non-Japanese biopharmaceutical companies looking to enter the Japanese market, the first challenge is often finding a comprehensive list of biopharma companies in Japan. While big names like Takeda or Astellas are well-known globally, many smaller, innovative players remain under the radar. These small-to-mid-size companies can offer unique partnership opportunities, but their visibility to international firms is limited.
One might expect Japan’s regulatory authority, the PMDA (Pharmaceuticals and Medical Devices Agency), to provide a list of biopharma companies. However, no such publicly available resource exists. This leaves international firms navigating a fragmented landscape, often relying on word of mouth or fragmented data.
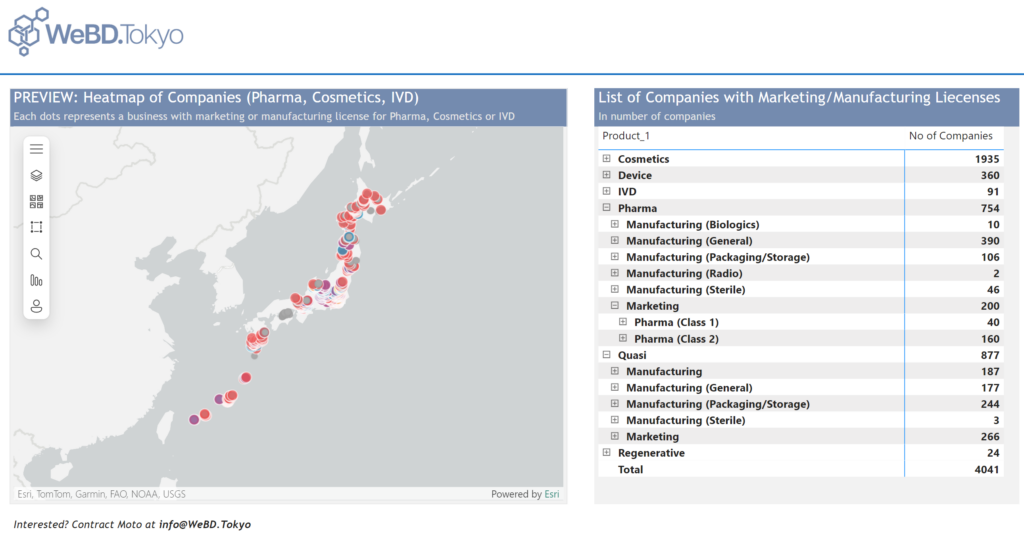
Recognizing this gap, WeBD.Tokyo has taken the initiative to create a comprehensive list of biopharmaceutical companies in Japan, leveraging the power of Artificial and Business Intelligence (ABI). This tool will help non-Japanese companies discover not only the major players but also the smaller, high-potential businesses that could be perfect for partnerships or collaborations. By offering detailed insights and profiles, WeBD.Tokyo’s database promises to make the Japanese biopharma landscape more accessible, connecting businesses and fostering innovation across borders.
The list includes:
- Marketing license (Class 1) for pharmaceuticals
- Marketing license (Class 2) for pharmaceuticals
- Marketing license for pharmaceuticals
- Marketing licenses for cosmetics
- Manufacturing license for pharmaceuticals
- Manufacturing license for quasi-pharamceuticals
- Manufacturing license for cosmetics
Are you interested to get the full list?
Feel free to send us a message through Contact.
By Prefecture
- Hokkaido
- Aomori
- Akita
- Tochigi
- Kyoto
- Kagawa
- Kagoshima
- Okinawa
- Tokyo
- Kanagawa
- Saitama
- Rest of prefecturers are in preparation
0 Comments